Transmission Electron Microscope
In a Transmission Electron Microscope (TEM) a beam of energetic electrons is focused on the sample to examine and image this one. Electrons are extracted from a cathode that is a tungsten filament heated to high temperature (1.550 to 2.700 ° C) and accelerated by application of high voltage current varying from 50.000 to 300.000 volt (at 80.000 volt the speed of electrons is 150000 Km/sec e.g., half the speed of light). The electrons that are very small must move in a column in which high vacuum (10-7 – 10-10 Torr) is maintained by elaborate pumps. This is required to allow electrons to produce an image without collision with any air molecules which may deflect them. The resolution of a modern TEM is about 0.2 nm and the can vary from 500 to 5.000.0000x according to the microscope.
Different electromagnetic lenses influence the electron beam. The first one is the condenser lens which focus the beam onto a very thin sample. The image-producing system consists of the objective lens, intermediate and projector lenses, which focus the electrons passing through the specimen to form a real, highly magnified image. Finally, we find the image recording system which usually converts the electron image into an image perceptible for human eye. It consists of a fluorescent screen for viewing the image and a digital CCD camera for their permanent records. A few pictures illustrate the FEI TECNAI TM G2 Sphera used for my research activities and collaborations at the Geneva University.
As result of the high vacuum inside the column it is not possible to observe living specimen and this involves different steps of their preparation. Most generally the samples are fixed, dehydrated and embedded in hard resin. Another constraint of the TEM is that samples must be very thin to allow electrons to pass right trough the sample. To reach this objective very thin sections named ultrathin sections (~ 60 nm thickness) are made using an ultramicrotome and a diamond knife (from a rough diamond) trough samples included in hard resin. The sections are deposited on a support that is a grid of 3,05 mm in diameter, the standard size for all TEMs. Before observation, they are contrasted with heavy metals aqueous, uranyl acetate and lead citrate (see protocols). These metals link preferentially on some cellular compounds which will scatter or absorb electrons creating dark areas. Parts of the specimen on which metals do not link transmit electrons and appear brighter.
Particular methods for staining and localization of different cellular compounds have also been routinely used (polysaccharides, proteins, lipids, enzymes….). A poster in the main entrance of Sciences III building, University of Geneva resumes the importance of this microscopy for sciences.
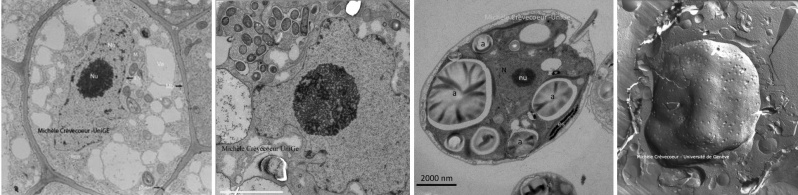
A series of electron micrographs of ultrathin sections taken during my activities at the university are shown below as well as another pages of this website, in particular on the pages of my research collaborations on Chlamydomonas, Arabidopsis, and on nodules induced on roots of legumes by Rhizobium sp. NGR234.
On other pages you will find electron micrographs illustrating different cells/ tissues. . Root cells in a ungerminated embryo – Zea mays . Cells from foliar parenchyma – Arabidopsis thaliana . Different cells/tissues from birch (Prunus avium) : stomata, xylem, epidermis, mesophyll.
See also the page devoted to Cryofracture a particular technique and the micrographs of replica of fractured primary roots and shoot meristems.
See the galery of Transmission Electron Micrographs of plant cells