Scanning Electron Microscope
In the Scanning Electron Microscope, a beam of high – energy electrons is emitted from an electron gun and accelerated down to an energy up to 30 keV. It passes through a combination of magnetic lenses and apertures that focus the electrons into a small probe. This rapidly scans the surface of the sample. The interactions between electrons and the sample surface produces various signals detected by appropriate detectors which allow to obtain information on the surface topography but also on compositional features of the sample. The resolution of modern SEM is about 10 nm, varying from 1 to 20 nm, and magnifications of 200.000x up to 1.000.000 x can be achieved. The SEM permits non-destructive examen of the specimen.
As for TEM a vacuum is required in a SEM, to protect filament and to avoid collision between electrons and gas molecules. To improve quality of images the surface of specimen is coated with a thin layer of metal, generally gold. The penetration of electrons in the matter is very low, particularly after coating.
Some biological samples relatively dry can be observed directly without metallization: seeds, pollen grains. On the contrary soft and hydrated samples need a particular preparation including chemical fixation, washing, dehydration, drying and finally metallization.
Exemple of protocol of specimen preparation
All the steps of the protocol take place at laboratory temperature.
● 1st fixation: 2.5% glutaraldehyde in sodium cacodylate buffer (0.1M; pH 7.0) for 2h
● Washing in buffer cacodylate 4 x 15 min
● 2nd fixation : 1% OsO4 in cacodylate buffer for 2 h
● Washing in Cacodylate buffer 2 x 15 min then with milliQ water 2 x 15 min
● Dehydration in ethanol :
Ethanol 30 20 min
Ethanol 50 20 min
Ethanol 70 20 min
Ethanol 90 20 min
Ethanol 100 I 3 x 20 min
● Critical air drying
● Metallization : coating with a thin layer of gold using a « JEOL 1200 – Fine coater » from the Bioimaging Platform, Sciences Faculty, Sciences II, University of Geneva.
● Observations: Jeol JSM-6510 LV microscope – see the Protocol for using – written in collaboration with Stéphane Hagmann (research auxiliary from May to August 2011).
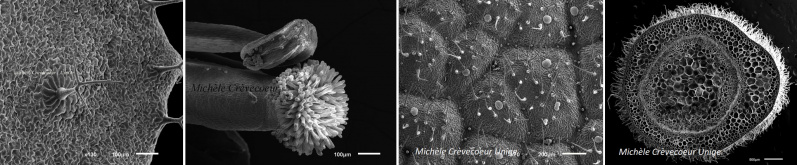
Plant organs imaged with the Scanning Electron Microscope JEOL JSM 6510LV
Imaging : Stéphane Hagmann (research auxiliary; Kilian Anderegg (apprentice); Michèle Crèvecoeur (Lecturer; Microscopist)
Annotations & picture’s comments : Michèle Crèvecoeur
Leaves :
Arabidopsis thaliana
Origanum vulgare
Rosmarinus officinalis
Salvia officinalis
Triticum durum
Drosera linearis
Stem :
Salvia officinalis
Roots:
Zea mays
Embryo:
Arabidopsis thaliana
Flower:
Arabidopsis
Scanning Electrons Microscopes in Geneva
Dr André Piuz
Geneva’s Natural History Museum
Scanning Electrons Microscopes’ laboratories and X-Ray microanalysis
Bioimaging Center
Faculty of Sciences (Sciences II) – Geneva University
Prof. Rossana Martini
Earth Science